Methods of Obtaining Experimental Data for the Construction of Phase Diagrams
The construction of a phase diagram of a multi-component system requires knowledge of the equilibrium phase relations of that system. This means establishing the phases existing at various temperatures for selected compositions within the system. The positions of invariant points, liquidus lines or surfaces, and other points, lines, or surfaces at which phase reactions occur must represent phase relations for equilibrium conditions. This can be especially difficult for many systems, such as the silicate systems, in which many metastable conditions exist and equilibrium phases are difficult to attain. Below is a list of important factors that must be considered in the determination of phase-equilibrium relations.
- Chemical purity of components. Starting materials should be as "pure" as possible since foreign impurities could possibly add unwanted additional components and phases in the system. With impurities present, the phase-equilibrium relations actually determined may be very different from the intended equilibrium relations. However, foreign constituents can be beneficial if they act as catalysts or mineralizers which increase the rates of reaction and prevent the formation of metastable phases. Today, starting materials range from 99.9% to 99.9999% purity for phase equilibrium studies.
-
Physical characteristics of Experimental Samples. Equilibrium is attained more easily when the area of contact of raw materials is greater. Thus, well combined fine-particled materials are desirable in these experiments. Starting materials can range from being in the form of finely ground powders to precipitated chemicals. In glass-forming systems, raw materials must be carefully examined to insure they are single phase and homogeneous.
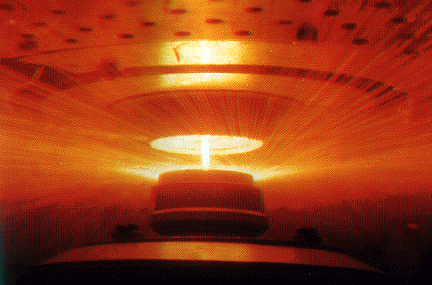
Figure 1 - A rapid solidification rate processing technique by which small powder particles of metal alloys are produced by very rapid cooling. A stream of molten metal is poured onto a disc that is rotating at a high angular velocity. Extremely small droplets of the metal are flung outward by centrifugal forces. The fast cooling rate is obtained by injecting jets of cold inert gas through the holes shown in the top of the apparatus (Callister, p. 429).
-
The time criterion. In the study of equilibrium relations, the time spent at any temperature must be long enough to allow the completeness of reactions. Although many solid state reactions and phase transitions occur in a matter of seconds, minutes, or hours, some can take years to reach full completion. In these cases, either the experiment must be carried out over years in order to obtain equilibrium, or the reaction must be accelerated by chemical or physical methods which do not affect the number of components or phases present in the original system under consideration.
-
Constancy of composition. Two major factors are involved in making sure the compositions of starting materials remain constant during experiments. The first is the container in which reactions occur, which varies for different reactions. Containers that will not react with the system under experimentation must be used The second important factor concerns equilibrium heat treatments in which volatilization of oxides occur, which can lead to compositions different from the starting mixtures. If phase equilibrium experiments are done with volatile oxides, the material should be heat treated in an enclosed container that will not react with the oxide composition and will prevent the oxides from leaving the system.
-
Chemical composition and chemical analysis. For equilibrium phase studies, the composition and purity of starting materials must be known. Compositions are identified through spectrochemical and chemical analysis. In some cases, chemical analyses of the final products are completed to ensure there was no change in composition during the course of the experiment.
With the criteria listed above in mind, phase equilibria relations are determined through many techniques, each falling under one of the following categories:
- I. Static Methods. Static methods are those in which the temperature of the sample is held constant until equilibrium is attained.
- II. Dynamic Methods. These methods are used when phase equilibrium determination at other than ambient conditions is required.
Once phase equilibrium relations are established, it is necessary to identify the phases present in the specimen. The primary methods of phase identification are the following:
These methods account for over 95% of direct phase identification. Other techniques include: (you can find a discussion on these topics in Volume I of Phase Diagrams: Materials Science and Technology, edited by Allen M. Alper, or in any book about phase diagrams)
- Mossbauer spectroscopy
- infrared spectroscopy
Susana Castro 4/28/96
http://www.eng.vt.edu/eng/materials/classes/MSE2094_NoteBook/
96ClassProj/experimental/tools.html
Project Hompage | Experimental Page | Reference Page
