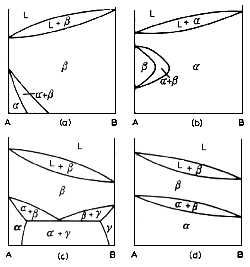

Above are some examples of diagrams that exhibit allotropy. Allotropy occurs when one metal exhibits more than one crystal structure. Examples are Titanium, with two, Iron, with three, and Plutonium, with six (yes, six). There are basically two classifications as far as diagrams are concerned. There are systems in which one phase is in equilibrium with the liquid phase, and systems in which two phases are in equilibrium with the liquid phase. The diagrams above refer to the former case. The first two on the left are examples of one component having allotropic configurations, the two on the right when both components have allotropes.
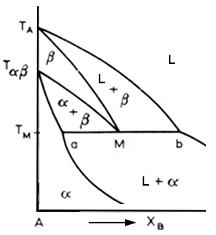
In this reaction, alloy of composition M, the metatectic composition, precipitate beta a they cool from the liquid phase. This beta content increases until it is all beta. But it is solid only for a short time, for when it reaches point M, it partially remelts. As cooling continues, the alpha phase increases until the solution is all solid and all alpha.
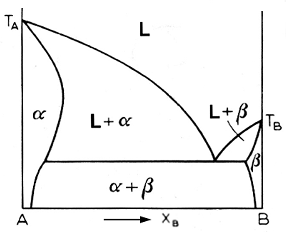
what we have above is an example of retrograde solidus curve. The curve bulges out and means that maximum solubility occurs somewhere between the melting point of the solvent and the eutectic temperature. Retrograde solidus curves are associated with high enthalpies of solution of the b component. High values for this quantity correlate with a big difference between the atomic radii of the two components, which lead to a high strain energy. Note that retrograde solubilities are associated with low solubilities in general.
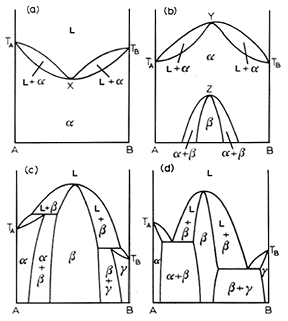
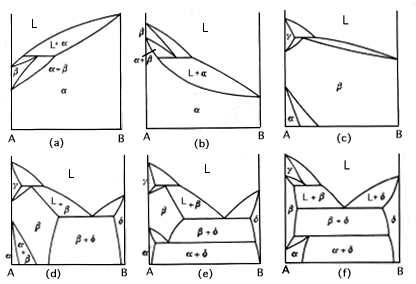
Congruent transformations are ones with a compositional alteration. Examples of congruent transformations include allotropic transformations and the melting of pure metals. So eutectic and eutectoid reactions are examples of incongruent transformations. The first and second diagram above from the left represent a melting point maximum and a melting point minimum congruent transformation. the latter two diagrams on the right represent congruent transformations with the formation of an intermediate phase. sometimes intermediate phases are classified according to whether they form under a congruent transformation.
http://www.eng.vt.edu/eng/materials/classes/MSE2094_NoteBook/
96ClassProj/analytic/simk1.html