by Paul Myslinski (resume). Modified by R.D. Kriz (5/18/95)
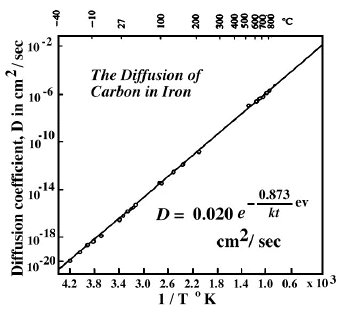
The most accurately known diffusion coefficient in solids is that for the diffusion of carbon atoms in body-centered cubic iron. By use of a variety of experimental techniques, measurements were made from -35°C to 800°C; the diffusion coefficient varies by fourteen orders of magnitude over this temperature range. When a plot of D vs. 1/T°K is formulated, D0 and Q can be computed from the intercept and slope of the line.

Figure 1. Diffusion Coefficient of Carbon in Iron as a function of Temperature
The resulting temperature dependence of the diffusion coefficient is given by:
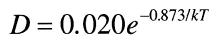
where D is the diffusion coefficient given in cm2/sec and the activation energy, in electron volts.
The motion of carbon atoms in an iron crystal is an example of interstitial diffusion in a body-centered cubic structure.
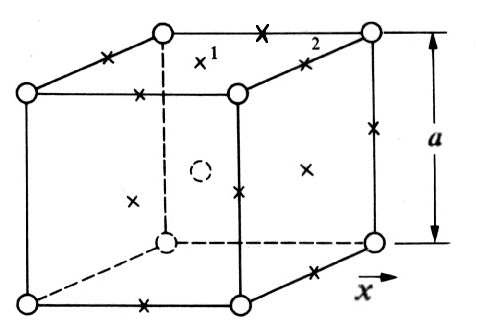
Figure 2. Location of Carbon interstitials
The interstitial positions that can accommodate a foreign atom (carbon), marked by x's, are at the centers of the faces and the mid point of the edges in the unit cube of the bcc structure in Fig. 2.
For diffusion in the x-direction, it is clear that an interstitial atom can contribute to diffusion only if it is at a face center, since if it at an edge, its motion in the x-direction is prevented by the presence of a host atom. The probabilities that an edge and the face-center positions have equivalent surroundings. We now ask the question, "If an interstitial atom is in a given plane, what is the probability that it will contribute to diffusion?" To answer this, choose the given plane to be that which is perpendicular to the x-direction and bisects the cube. There are eight interstitial positions in this plane that are on the unit cube. You may want to look at Fig. 2 again to observe the locations of these interstitials.
Four of these positions are at face centers and four are at edges. Now each face-center position is shared by one other cube, so there are a total of two face-center positions per unit cube in the crystal. But each edge position is shared by four cubes, so there is just one edge position per unit cube. Since there are a total of three interstitial positions per cube, and since the two positions at the face centers can diffuse, the probability that an interstitial atom in a given plane can contribute to diffusion is 2/3. This number is just the constant "alpha" in the equation shown below.
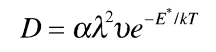
for the bcc structure so that in this case the diffusion coefficient is:
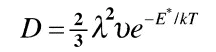
The jump distance "lambda" is just one half the cube edge, which we will call a, so
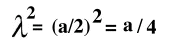
and the previous equation can also be written as
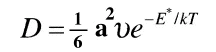
The following figure shows the arrangement of nearest-neighbor host atoms for two adjacent interstitial positions 1 and 2. Position 1 is surrounded by six atoms, four in a plane perpendicular to the paper and two in the plane of the paper.
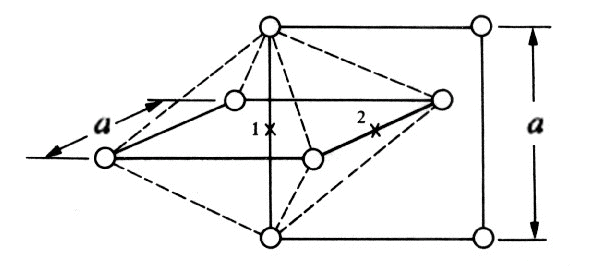
Figure 3. Alternate view, geometry, of interstitial carbon sites
The application of a little plane geometry shows that these six atoms are not equidistant from position 1; the two atoms in the place of the paper at a distance a/2 from the interstitial position whereas the other four atoms are at a distance a*sqrt(2)/2 . The six atoms are at the corners of an octahedron which is shown by the dotted lines in the figure, and therefore the interstitial position in the center of this octahedron is frequently referred to as an octahedral position
From the previous diagram, we see that position 2 also sits in the center of an octahedron and that the atomic arrangement around it is the same as around position 1 except that the four atoms at a*sqrt(2)/2 are in the plane of the paper while the atoms at a/2 are on a line perpendicular to the paper.
The two sites, therefore, have the same surroundings, except for orientation, and are energetically equivalent.
Now if we place a foreign atom at position 1, the host atoms will be pushed away from their original positions because of the strong repulsive forces that act between atoms when they are brought so close together.

Figure 4. Mr. Myslinski, we need a figure here, contact me at rkriz@vt.edu
This situation shows the atomic arrangement when the interstitial is at site 2. Since these two configurations are exactly equivalent, the energy of the crystal is the same no matter which of the two sites contains the interstitial atom.

Figure 5. Mr. Myslinski, we need a figure here, contact me at rkriz@vt.edu
During a diffusion jump, the interstitial atom moves from one octahedral site to another. Midway between these two sites, it is surrounded by four atoms all at equal distances from the foreign atom. The four atoms form a tetrahedron with the foreign atom at the center. This tetrahedral site has a barrier to the motion of the interstitial atom. The tetrahedral configuration is the activated state for the jump, and the system must acquire an activation energy to overcome the energy barrier.
The thermal fluctuation that supplies the activation energy can have several effects. In the first place, it can supply so much momentum, in the proper direction, to the interstitial atom that it drives its way through the tetrahedral position, forcing the adjacent octahedral position to open up to accept it. Also, a fluctuation can take place in which the atoms around the empty octahedral site move away from it in phase so as to open up a hole into which the foreign atom can fall even if its energy is not much greater than the mean value. Of course, combined fluctuation, in which these two effects have varying relative importance, also can supply the needed activation energy. We have no way of knowing which of these two effects is favored in actual diffusion jumps, but some theoretical calculations have been made which indicate that the "opening up" effect is the important one. By calculation the energy required to push the iron atoms surrounding the empty site far enough apart to receive the carbon atom, a theoretical value for the activation energy is obtained that agrees reasonably well with experiment.
End of Section on Diffusion of Carbon in Iron: some ideas not found in your text